An Oct 2021 paper in BMJ Nutrition Prevention and Health explored the relationship between fruit and vegetable intake and mental wellbeing in school children[i]. This timely research, bearing in mind the progressive increase in childhood mental health problems, highlighted the direct association between fruit and vegetables consumed over breakfast and lunch and their related impact on mental wellbeing.
Just as in prior national studies looking at food choice, this study exposed that of the 9,000 Norfolk based school children, only 28% ate the recommended five a day of fruit and veg, and 10% ate none. Those that consumed energy drinks for breakfast fared the worst!
No surprise to the passionate advocates of recognising nutrition as a modifiable factor in individual and societal development, but still well off the radar of many schools, parents and public health focus.
Metabolic Challenges and the Gut
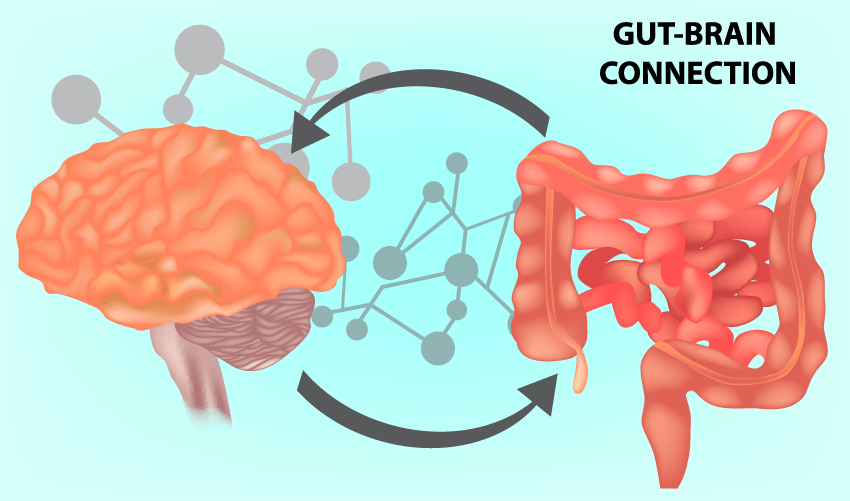
But why stop there? It is well known that an unhealthy lifestyle is a major risk factor for metabolic diseases, while in recent years, accumulating evidence has demonstrated that the gut microbiome and its metabolites also play a crucial role in the onset and development of many metabolic diseases, including obesity, type 2 diabetes, non-alcoholic fatty liver disease, cardiovascular disease and so on. They are also associated with brain function, mental well-being and focus[ii].
Intestinal metabolic health status is relayed to the brain by both neural and circulatory pathways. Bidirectional gut-brain neural relays control satiety signalling and appetite regulation. Detection of luminal metabolites by intestinal enteroendocrine cells is communicated to the brain via activation of the vagus nerve, by epithelial cell-derived hormones and neurotransmitters, and via neural routes, including neuroepithelial connections.
Dietary metabolites and epithelial-derived soluble factors may also travel via the bloodstream to the Blood Brain Barrier, where a variety of nutrient transporters and specific receptors expressed on brain microvascular endothelial cells facilitate the translocation of specific nutrients (e.g. glucose, lactate) across the barrier into the brain parenchyma, and allow for binding of circulating peptide hormones and other factors which can modulate brain activity.
The microbial metabolites discussed above, including short chain fatty acids, Trimethyamines, amino acid metabolites, and vitamins can travel via or modulate these pathways to affect enteroendocrine, neuronal, and immune cells in the gut, liver, kidneys, pancreas, and brain. In effect, what you eat, and how you metabolise it has direct and indirect impact on all organ systems in the body.
Immune and Neurotransmitter Signalling
 In addition to their actions on enteroendocrine and neural cells, metabolites can act via G Protein coupled receptors and other receptors to direct the development and normal functioning of the mucosal immune system; regulating the recruitment, development, maintenance, and activation of immune cells. The balance of dietary and bacterial metabolites is important for maintaining immune tolerance, and in directing inflammatory responses in the gut.
In addition to their actions on enteroendocrine and neural cells, metabolites can act via G Protein coupled receptors and other receptors to direct the development and normal functioning of the mucosal immune system; regulating the recruitment, development, maintenance, and activation of immune cells. The balance of dietary and bacterial metabolites is important for maintaining immune tolerance, and in directing inflammatory responses in the gut.
Fixing the Problems
Disruption of the complex ecosystem of the intestinal microbiota is now implicated in multiple conditions and diseases of both the intestine and the brain, and some of the routes by which microbes may act on the gut-brain axis are beginning to be better understood. Manipulating or shaping the microbiota is therefore considered a usable strategy to prevent or treat various extra-intestinal diseases and enhance brain function. The beneficial effects of an ‘optimised’ gut microbiota includes immune and epithelial homeostasis, enteric nervous system regulation, and optimal digestion and metabolism with reduction in metabolic dysfunction and related disease.
FODMAPS
Loss of function in the gastro-intestinal tract, is pervasive and common. Irritable bowel syndrome is modulated by diet and modification of the microbiome. Whilst still in exploration, the role of probiotics in the assistance of recovery of function continues to gain credibility and expert application of food selection enhances outcomes. The correct application of the low FODMAP diet also appears to offer benefit but requires careful application to avoid unintended immune deficits in the mucosal epithelium. Clinical wisdom is required in utilising the low-FODMAP diet[iii].
What to Do?
Current therapeutic approaches include modifying the existing microbial composition in the gut by: altering nutrient availability (increased fibre via plants and fruits) to promote the growth of particular classes or species of bacteria (prebiotics); These include specific fibre extracts (often in food supplements) such as disaccharides (lactulose), oligosaccharides (fructooligosaccharides, FOS, galactooligosaccharides, GOS) and polysaccharides (inulin). Other compounds that can also be considered as prebiotics are resistant starches, soluble fibre, pectin, whole grains, and polyphenols[iv],[v].
Obviously introducing or expanding ‘beneficial’ species by the inclusion of probiotics; or by wholesale transplant of entire communities or portions of communities from other intestinal donors (faecal microbial transplantation and more selective stool transplants) also have a well-established role[vi],[vii].
Nutritional therapy, including the guidance on specific compounds, whole food groups and the application of specialised therapeutic interventions such as alternative fasting, food stratification and food exclusion, offers simple, safe and effective opportunities to apply one to one strategy[viii]. Public policy may take a long time to evolve, but the realisation that we are killing people in ‘metabolic slow motion’ by avoiding the changes needed to food production and consumption, will, like climate change eventually attract action.
References
[i] Hayhoe R, Rechel B, Clark AB, et al Cross-sectional associations of schoolchildren’s fruit and vegetable consumption, and meal choices, with their mental well-being: a cross-sectional study
BMJ Nutrition, Prevention & Health 2021;e000205.
[ii] Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11(2):135-157.
[iii] Hill P, Muir JG, Gibson PR. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol Hepatol (N Y). 2017;13(1):36-45.
[iv] Edwards PT, Kashyap PC, Preidis GA. Microbiota on biotics: probiotics, prebiotics, and synbiotics to optimize growth and metabolism. Am J Physiol Gastrointest Liver Physiol. 2020 Sep 1;319(3):G382-G390.
[v] Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Woźniak K, Aprotosoaie AC, Trifan A. Bioactivity of dietary polyphenols: The role of metabolites. Crit Rev Food Sci Nutr. 2020;60(4):626-659.
[vi] Wolter M, Grant ET, Boudaud M, Steimle A, Pereira GV, Martens EC, Desai MS. Leveraging diet to engineer the gut microbiome. Nat Rev Gastroenterol Hepatol. 2021 Sep 27.
[vii] Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, Xu XY, Li HB. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients. 2021 Sep 15;13(9):3211.
[viii] Riccardi G, Rivellese AA. Dietary treatment of the metabolic syndrome–the optimal diet. Br J Nutr. 2000 Mar;83 Suppl 1:S143-8.
